Which of the Following Best Describes an Arrhenius Acid-base Reaction
It accepts a lone pair of electrons. Which of the following best describes an Arrhenius acid-base reaction.
What Type Of Reaction Occurs When An Arrhenius Acid Reacts With An Arrhenius Base Quora
Acid base H OH- O C.

. Acid base H20 B. For the reverse reaction NH 4 is the acid it donates H to become NH 3 and OH. Acid base conjugate base conjugate acid O C.
NaOH aq Na aq OH aq Some other examples of Arrhenius base are 1st and 2nd group hydroxides like LiOH and Ba OH2. It donates a proton. Hence Arrhenius acid-base reaction is the combination of an acid and base to produce salt and water as products.
Acid base H20 B. Which of the following correctly describes an Arrhenius acid. Acid base H.
3 on a question Which of the following best describes an Arrhenius acid-base reaction. For the following reaction label each substance as an acid or a base and show the conjugate acid-base pairs. Furthermore Arrhenius stated that this two ions combine in an aqueous solution to give water.
It produces OH in solution. Hence Arrhenius acid-base reaction is the combination of an acid and base to produce salt and water as products. Acid base salt water.
3 on a question Which of the following best describes an Arrhenius acid-base reaction. First one is the correct answer. What equation best describes an Arrhenius acid base reaction.
NH 3 H 2 O NH 4 OH-Solution In the forward reaction NH 3 is the base it accepts H to become NH 4 and H 2 O is the acid it donates H to become OH-. Acid base conjugate base conjugate acid. Previous Next.
L i O H s H 2 O L i a q O H a q LiOH s In the above reaction lithium hydroxide dissolves in water to give lithium-ion and hydroxide ion. Acid base H OH- C Acid base salt water D. Acid base salt water What is equation that describes the reaction between water and hydrochloric acid.
Brønsted-Lowry base because in the chemical reaction the CO3 compound in the reactant receives a Hydrogen ion in the product. This is a neutralization reaction. Acid base H20 O B.
Acid base salt water B. Narendra modi is currently the pm of india. Acid base H OH O D.
Which of the following describes an aqueous solution of ammonium sulfate. Question 7 of 25 Which of the following best describes an Arrhenius acid-base reaction. Acid base salt water.
In accordance to the proposal of a scientist called Svante Arrhenius an acid is a substance that increases the H concentration of a solution by dissociating into it while the base increases the OH- concentration of a solution by dissociating into it. - 16155652 kyleroberts610oy97pv kyleroberts610oy97pv 05042020 Chemistry Middle School answered Which of the following correctly describes an Arrhenius acid.
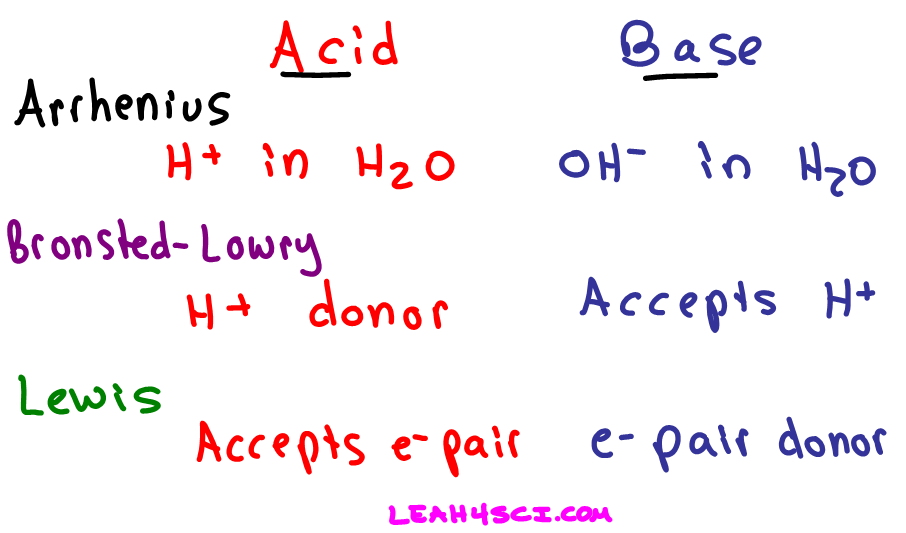
Definitions Of Arrhenius Bronsted Lowry And Lewis Acids And Bases In Organic Chemistry

Which Of The Following Best Describes An Arrhenius Acid Base Reaction Brainly Com

Follow Mxxnlightprincess For More Video School Organization Notes Science Notes School Study Tips
0 Response to "Which of the Following Best Describes an Arrhenius Acid-base Reaction"
Post a Comment